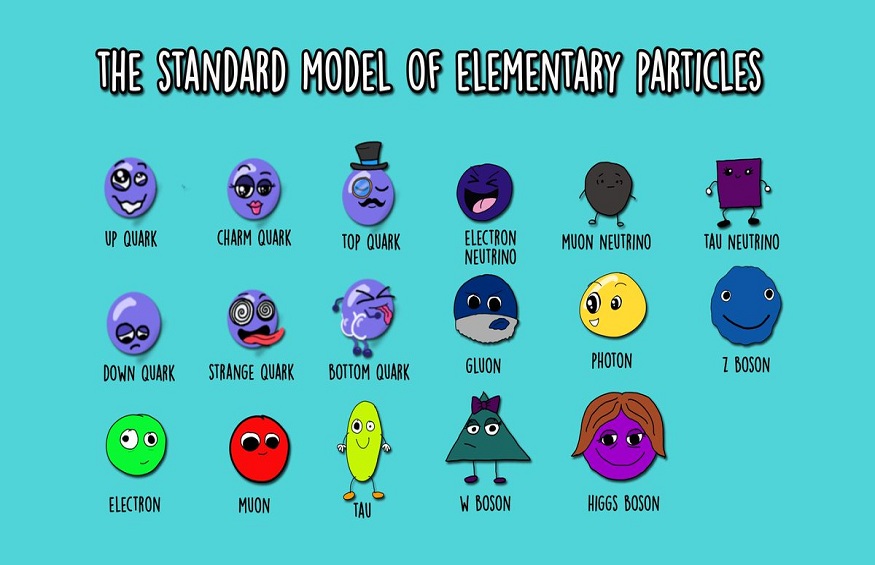
Atom and elementary particles are the tiniest known building blocks of the universe. They do not possess any inherent structure, symbolising that researchers assume them as zero-dimensional points that take up no space. Electrons are apparently the most common elementary particles. The Standard Model of physics represents the interactions of particles and almost all forces, comprising 10 total elementary particles.
Atom and it’s model
An atom is the smallest unit of matter. An atom comprises these elementary particles. Each and every atom has its own unique properties and structure but the basic model of the atom remains the same. The first model of the atom was proposed by Rutherford and later Neils Bohr modified his model of atom. Neils Bohr was the son of a popular physician, Christain Bohr who framed a theory called the Bohr Effect. Neils Bohr predicted his model of the atom in the year 1915. Neils Bohr stated that “electrons in atoms travel around a central nucleus in circular orbits and can only orbit stably at a distinct set of distances from the nucleus in certain fixed circular orbits”. His model of the atom is based on Planck’s theory of quantization.
Composition of atom
Atom is the smallest indivisible unit of an element. An atom is an important part of all substances as it defines the properties of the substances. An atom is made up of various subatomic particles called protons, electrons and neutrons. The discovery of subatomic particles has been the basis and platform for many other huge discoveries and inventions. A lot of research work has been done to demonstrate the composition of an atom by various scientists.
What are elementary particles?
Elementary particles also referred to as fundamental particles are subatomic particles that cannot be broken down into further sub-structures. These particles are not built by any other particles. Elementary particles can be considered as the fundamental constituents of all substances (matter and antimatter). Example: The fundamental fermions such as leptons, quarks, antileptons, and antiquarks are a vital example of elementary particles. These particles make an atom.
Discovery of elementary particles
The first subatomic particle discovered was the electron in 1897 by J. J. Thomson. Then follows the discovery of the proton by Ernest Rutherford in 1911. Later in 1932 Neutron was discovered by British physicist Sir James Chadwick. Atoms of various elements have different atomic structures as they possess different numbers of protons and electrons. This stands the reason for the unique characteristics of all the elements. After these discoveries, many scientists proposed various models of the atom based on their research and studies.
Conclusion
Atom and elementary particles are the fundamental units for the entire universe. Many concepts, relations, equations, theories and graphs have been discovered based on them. They play a crucial role in the study of concepts in subjects such as physics and chemistry. Many scientists and researchers have been awarded Nobel Prizes for their contributions in Science and also in the advancement of technology. Much research is still in progress for in-depth study of these particles and matter.